Our research is based on the following subjects:
1. Regeneration, Differentiation, and Proliferation of Pancreatic beta cells (Jamileh Movassat)
We are currently interested in the role of the Wnt/beta-catenin and Notch/Delta signalling pathways in regulating beta-cell proliferation and neogenesis in normal conditions as well as during the course of situations of compensated growth required to confront the increased need of the organism for insulin in certain physiological (gestation) or pathological (insulin resistance) contexts. Our group is expert in studying beta-cell regeneration and in developing innovative approaches aimed at inducing in situ regeneration of beta cells, especially by activating differentiation of intrapancreatic precursors into beta cells.
2. Impaired insulin exocytosis during type 2 diabetes (Cécile Tourrel-Cuzin)
The pathophysiology of reduced insulin secretion in type 2 diabetes remains badly understood but various clinical experiments and arguments involve distal stages of insulin exocytosis by the pancreatic beta cells. These distal stages involve two partners: the actin cytoskeleton in the subcortical region and the SNARE proteins that regulate the accosting of the granules secreted and their fusion with the plasma membrane in response to an external stimulus such as glucose. Interactions between the actin filament network and the SNARE proteins are crucial in the optimal activation of this exocytosis in response to glucose.
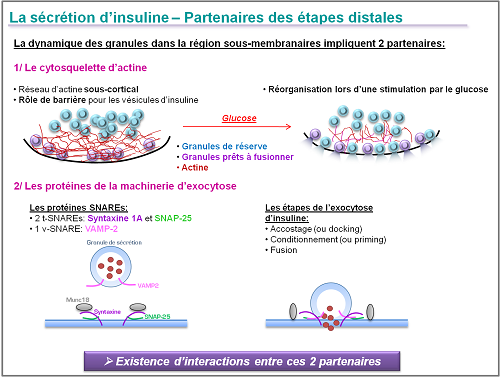
The aims of our research are the following:
1/ Understanding the molecular mechanisms controlling the insulin exocytosis
In using a combination of molecular and cellular approaches (living cell imaging – TIRFM) we can research into the expression and functionality of the main molecular actors that regulate exocytosis and insulin. In particular, we focus on the interactions between the SNARE proteins and the subcortical actin network in healthy or diabetic pancreatic beta cell. This approach means we can understand the etiology of exocytosis malfunction in the pancreatic beta cell subject to a diabetic environment.
2/ Therapeutic cellular approaches to correcting exocytosis malfunction in the β cell
We have recently shown in vitro that it is possible to restore the secretion of insulin in response to glucose thanks to acute exposure of the GK islets to GLP-1, activator of the AMPc dependent signalling pathways (Dolz et al, Diabetes 2005). We have therefore set up a project researching into the influence of the AMPc PKA dependent and/or Epac2 dependent pathways on the distal stages of insulin exocytosis in pancreatic beta cells.
3.a Energy metabolism and intracellular signalling in pancreatic beta cell in type 2 diabetes (MH Giroix)
Dans des modèles de DT2 induit (rat n0-STZ) ou spontané (rat GK), nous avons été parmi les premiers à mettre en évidence comme facteurs causaux du dysfonctionnement sécrétoire des cellules beta plusieurs perturbations métaboliques intrinsèques des cellules insulaires (e.g. glycolyse aérobie et production d’ATP, métabolisme et composition en acides gras des phospholipides altérés). Les recherches se poursuivent et diverses approches pharmacologiques sont testées pour contrecarrer les anomalies détectées et ainsi améliorer/restaurer la sécrétion d’insuline.
b/ Emergence du DT2 et mise en place de systèmes d’adaptation/protection chez le rat GK
L’analyse du rat GK a été entreprise à différents stades de son développement (1) fœtus (soumis à l’hyperglycémie/hyperlipidémie maternelles), (2) nouveau-né prédiabétique (normoglycémique), (3) adulte diabétique (hyperglycémique). D’ores et déjà, il ressort que toute une série de paramètres sont altérés chez le rat GK avant l’apparition des signes cliniques du DT2. Notamment, les fœtus et/ou nouveau-nés présentent: (1) au niveau systémique, une hypercholestérolémie (facteur reconnu de risque athérogène) et des marqueurs d’inflammation, (2) au niveau pancréatique, un défaut de vascularisation et des signes de microangiopathie et d’angiogénèse déficiente, ce qui a certainement un impact négatif sur non seulement le développement des îlots et la masse beta cellulaire mais aussi sur leur maturation fonctionnelle; (3) au niveau du foie: une altération de l’expression génique et l’activité de protéines impliquées dans le métabolisme du cholestérol (collaboration avec N. Janel dans l’équipe 5 (Resp. J.-M. Delabar) de BFA. Nos données orientent vers la piste du rôle déterminant d’anomalies précoces affectant le métabolisme lipidique hépatique et la vascularisation des îlots pancréatiques dans la programmation in utero et/ou l’installation du DT2 chez les rats GK. Elles révèlent aussi la mise en place, de différentes manœuvres (e.g. surexpression pancréatique de gènes/protéines Reg) pour lutter contre l’environnement diabétique délétère.
4. Glucolipotoxicity and metabolic adaptation of beta cells to insulin. Insulin metabolism by Sphingolipid (Hervé Le Stunff)
The pathology of type 2 diabetes is characterised by malfunctioning of pancreatic beta cell function and survival. The increased apoptosis of the β cells is due to their chronic exposure to strong concentrations of glucose. In the case of type 2 diabetes associated with obesity, chronic hyperglycemia strengthens the harmful effects of excess free fatty acids on the beta cell. This phenomenon is called glucolipotoxicity.
Different mechanisms have been suggested to explain the harmful effect of glucolipotoxicity including the production of the bioactive sphingolipids, the ceramides. Analysis of the role of the sphingolipid in biology during the last ten years has been significantly accelerated by cloning of the genes encoding of the enzymes that metabolise these lipids as well as the development of new techniques for analysing lipids (lipidomics). Thanks to these recent advances, we are seeking to determine the role of the metabolism of the sphingolipids such as the ceramides and sphingosine-1-phosphate, and also their action mechanisms in pancreatic beta cells during onset of glucolipotoxicity.


