Ontogenesis of the gonadotrope axis
– Differentiation of the pituitary gonadotrope lineage (PI: David L’Hôte)
Gonadotrope lineage specification depends on complex signaling and transcriptional cascades taking place during embryogenesis, whose molecular mechanisms remain elusive.
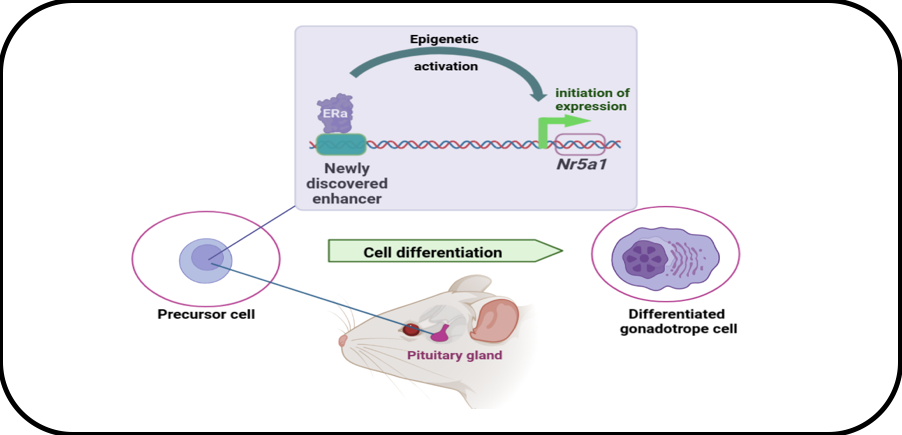
We have studied the regulation of the Nr5a1 gene encoding the most precocious factor involved in gonadotrope specification. This has been performed using a set of cell lines recapitulating gonadotrope cell differentiation and using cutting-edge functional genomic analyses such as chromatin accessibility assessment (ATAC-Seq), genomic editing (CRISPR/Cas9) and Chromatin Conformation Capture. We demonstrated that initiation of Nr5a1 expression is dependent on a novel enhancer, i.e. a distal cis-regulatory genomic region, which is activated transiently during pituitary development. We further showed that the enhancer activity is driven by estrogen alpha receptor (ERα), in agreement with the emerging role of ERα as a major chromatin regulator. Current research aims at understanding ERα mechanisms of action by identifying its partners in differentiating gonadotrope cells.
Role of ERa in the epigenetic mechanisms controlling Nr5a1 gene expression in immature gonadotrope cells.
DOI: 10.1186/s13072-019-0291-8
– Activation of the gonadotrope axis during minipuberty (PI: Céline Guigon)
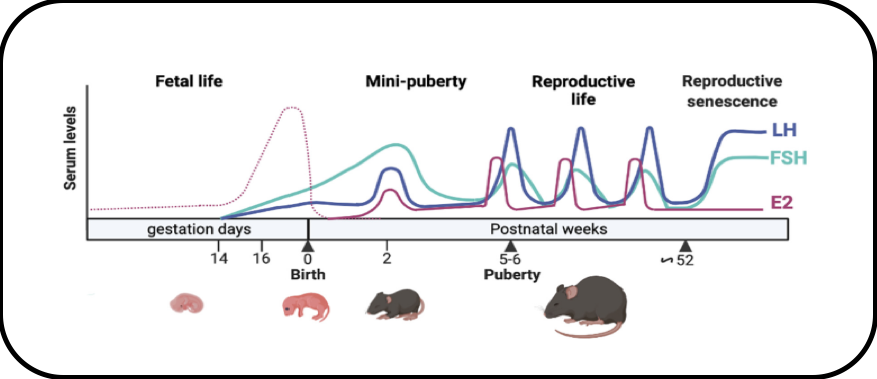
In female mammals, minipuberty of infancy is characterized by a transient and robust postnatal activation of the hypothalamo-pituitary-ovarian axis. We have performed in-depth analyses of the endocrine profiles during minipuberty in the mouse and studied the regulation of intra-ovarian production of estradiol. We provide novel insights on the mechanism by which the high levels of FSH at minipuberty stimulate estradiol production, notably through FSH-mediated down regulation of AMH, without affecting follicular growth.
Current research (ANR Reprofun funding) aims at assessing the possible role of minipuberty in programming female fertility.
This work is carried out in collaboration with S. Mhaouty-Kodja (Neuroscience Paris Seine, Sorbonne Université).
Activity of the gonadotrope axis throughout life in the mouse
DOI: 10.1530/JOE-18-0313
Regulation of the gonadotrope axis
– Characterization of the molecular mechanisms underlying gonadotrope cell plasticity during the estrus cycle (PIs: David L’Hôte and Ghislaine Garrel)
During the sexual cycle in adult females, pituitary gonadotrope cells exhibit a remarkable endocrine plasticity that orchestrates cyclic ovarian activity, and, hence, the coordinated activity of female reproductive organs. During each estrus cycle, this plasticity culminates to generate a massive release of LH and FSH, which is essential for female fertility as it triggers ovulation. The molecular mechanisms by which gonadotrope cells integrate all the regulatory signals responsible for their plasticity in vivo are far from elucidated, mainly due to the structural complexity of the pituitary gland.
We have thus performed single-nucleus RNA-seq analyses of rat pituitaries at different stages of the estrus cycle in order to elucidate the transcriptional changes taking place specifically within gonadotrope cells. Significant transcriptional variations were detected not only in gonadotrope cells but also in most endocrine and non-endocrine cell types, suggesting that the whole pituitary gland is implicated in the regulation of the estrus cycle. Focusing our attention on gonadotrope cells, we observed that transcriptional plasticity mainly takes place during pro-estrus day. We detected massive changes in the expression of unexpected gene families which are under current investigation.
This work is carried out in collaboration with F Miralles (Institut Cochin) and O. Taboureau (BFA).
– Regulation of ovarian folliculogenesis by estradiol (PI: Stéphanie Chauvin)
Whilst the importance of estradiol (E2) in follicle development is well established, the role of its nuclear receptors ERα and ERβ in granulosa cell (GC) biology remains incompletely understood, particularly in humans that possess five ERβ isoforms. We demonstrated that ERβ1, ERβ2, ERβ4 and ERβ5 are the predominant ERs in primary cultures of human GCs, and showed that E2 promoted a selective upregulation of the expression of some but not all ERβ isoforms. Our work also provides new findings in the field of estrogen signaling, in particular on the various roles of ERβ isoforms on cell fate, since we uncovered the pro-apoptotic activities of the ligand-sensitive ERβ1 and the ligand-insensitive ERβ4 isoforms in a human GCs line.
Therefore, our findings highlight a possible role of E2 to orientate GCs fate by specifically regulating the relative expression of ERβ isoforms. E2 would thus modulate the balance between pro-apoptotic and non-apoptotic ERβ molecules that could in fine control follicle selection.
This work is carried out in collaboration with N. Chneiweiss and A. Mayeur, Unit of Reproductive biology, CECOS, Hôp. A. Béclère.
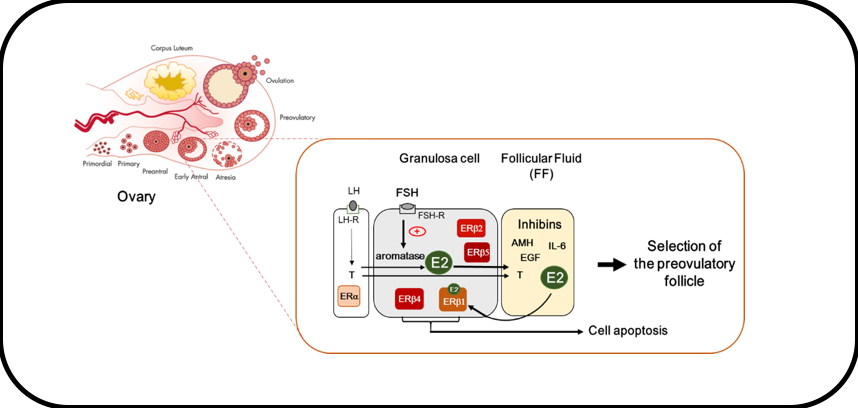
Working model of E2 action in GCs
In GCs of small antral follicles, FSH stimulates the expression of the aromatase that converts thecal-derived androgens (i.e., testosterone (T) produced upon LH stimulation) to estrogens (E2). E2 contained in the follicular fluid (FF) synergizes with FSH to promote follicular growth and further participates in the selection of the dominant preovulatory follicle. Contrary to thecal cells that principally express ERα, GCs mainly express ERβ isoforms. Among them, only ERβ1 binds to E2. ERβ1 as well as ERβ4 possess pro-apoptotic activities that may influence follicle fate.
DOI: 10.3390/ijms22095046
Vulnerability of the gonadotrope axis
– Metabolic disorders and pituitary gonadotrope activity (PIs: Ghislaine Garrel and Joëlle Cohen-Tannoudji)
Obesity, whose prevalence is increasing worldwide, negatively impacts reproduction. A low-grade inflammation is known to be associated to diet-induced obesity and hypothalamic inflammation was shown to disrupt the central control of energy homeostasis.
Given that we recently shown that fatty acids can be sensed by the pituitary and alter the synthesis of gonadotropins, we wondered whether pituitary inflammation contributes to alterations in gonadotropin secretion reported in obese patients and animal models of diet-induced obesity. In male rats fed a short- or long-term high-fat diet (HFD), we observed hypothalamic inflammation, as expected. HFD also led to a decrease in the synthesis and release of gonadotropins but interestingly, this was not associated with any increase in pituitary gene expression of inflammatory mediators. Diet supplementation with DHA, an omega 3 polyunsaturated fatty acid displaying anti-inflammatory properties, attenuated metabolic disorders and hypothalamic inflammation but did not prevent the disruption of gonadotropin secretion. Altogether, this work highlights a different inflammatory susceptibility of the pituitary to overnutrition and a high sensitivity of reproductive axis to diet.
Ongoing single-cell RNA-seq analysis (IdEx-funded Lipophyse project) should led new light on the molecular mechanisms protecting gonadotrope cells to nutrition-induced inflammation.
This work is carried out in collaboration with C. Magnan and O. Taboureau (BFA).
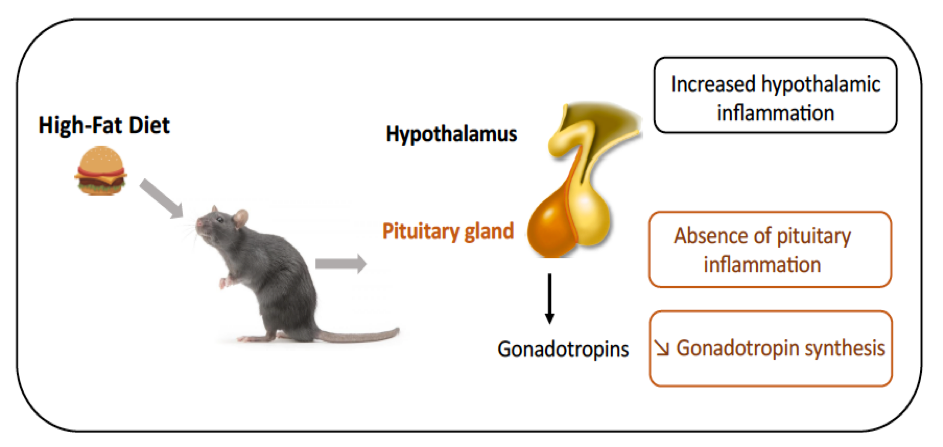 Consequences of high-fat diet on pituitary inflammation and gonadotrope activity
Consequences of high-fat diet on pituitary inflammation and gonadotrope activity
DOI: 10.3389/fendo.2022.877999
Estradiol and Polycystic ovary syndrome (PIs : Stéphanie Chauvin and Michaël Grynberg, Head of Departments of Reproductive Medicine of A. Béclère and J. Verdier Hospitals).
Estradiol (E2), locally produced by granulosa cells (GCs) of ovarian antral follicles, controls follicular development and selection, and triggers the gonadotropin surge that leads to ovulation. Dysovulation arising from the arrest of antral follicle growth and maturation occurs in up to 10 % of reproductive-aged women suffering polycystic ovary syndrome (PCOS). Although the diagnostic criteria for PCOS are clearly established, the molecular etiology of this syndrome is still under debate. In a recent comparative study in which we measured steroid concentrations in both GCs and follicular fluids from matched follicles of women with or without PCOS, we showed that contrary to previous dogma, GCs from PCOS follicles showed normal E2 biosynthesis. The decline of E2 concentration in PCOS follicular fluid would rather come from an increase of E2 degradation in matched follicular fluid.
In addition, in this study, we provide novel hallmarks in PCOS by revealing the impairment of E2 signaling in GCs of follicles from PCOS women that may have a negative impact on the emergence of dominant follicles, thereby contributing to their infertility
– Estradiol and granulosa cell tumors of the ovary (PI: Céline Guigon)
Granulosa cell tumors (GCT) are a rare and severe form of ovarian cancers, which displays a high risk of late recurrence. Since most patients present high levels of E2 we have investigated the roles and mechanisms of action of E2 on GCT growth through its nuclear receptors (ERα and ERβ).
Using selective ERα and ERβ agonists on human ERα- and ERβ-deleted GCT lines, we demonstrated that E2 promotes the growth of GCT cells mainly through ERα-nuclear-dependent mechanisms. E2 also induces GCT growth through ERβ-nuclear-independent pathways, but it required the presence of ERα. Given that we previously reported that E2 could inhibit GCT metastasis spreading through the G protein-coupled estrogen receptor (GPER), our work uncovered the versatility of E2 action in GCT, driven by the different forms of ERs.
 Versatility of the role of E2 on GCT growth depending on the of E2 receptor.
Versatility of the role of E2 on GCT growth depending on the of E2 receptor.
DOI: 10.1002/path.5843
The different axes of research all benefit from the technical support provided by three engineers: E. Airaud, R. Corre and A. Pierre. These three engineers are also involved in transversal activities within BFA Unit: E. Airaud is in charge of the FlexStation 3 facility of the Metabolism Platform; R. Corre is in charge of in situ technologies within BFA and is also one of the two Competent Radiological Protection persons of the Unit; A Pierre is the communication correspondent of BFA.